Bose-Einstein Condensation
EOS of Ideal Bose Gas
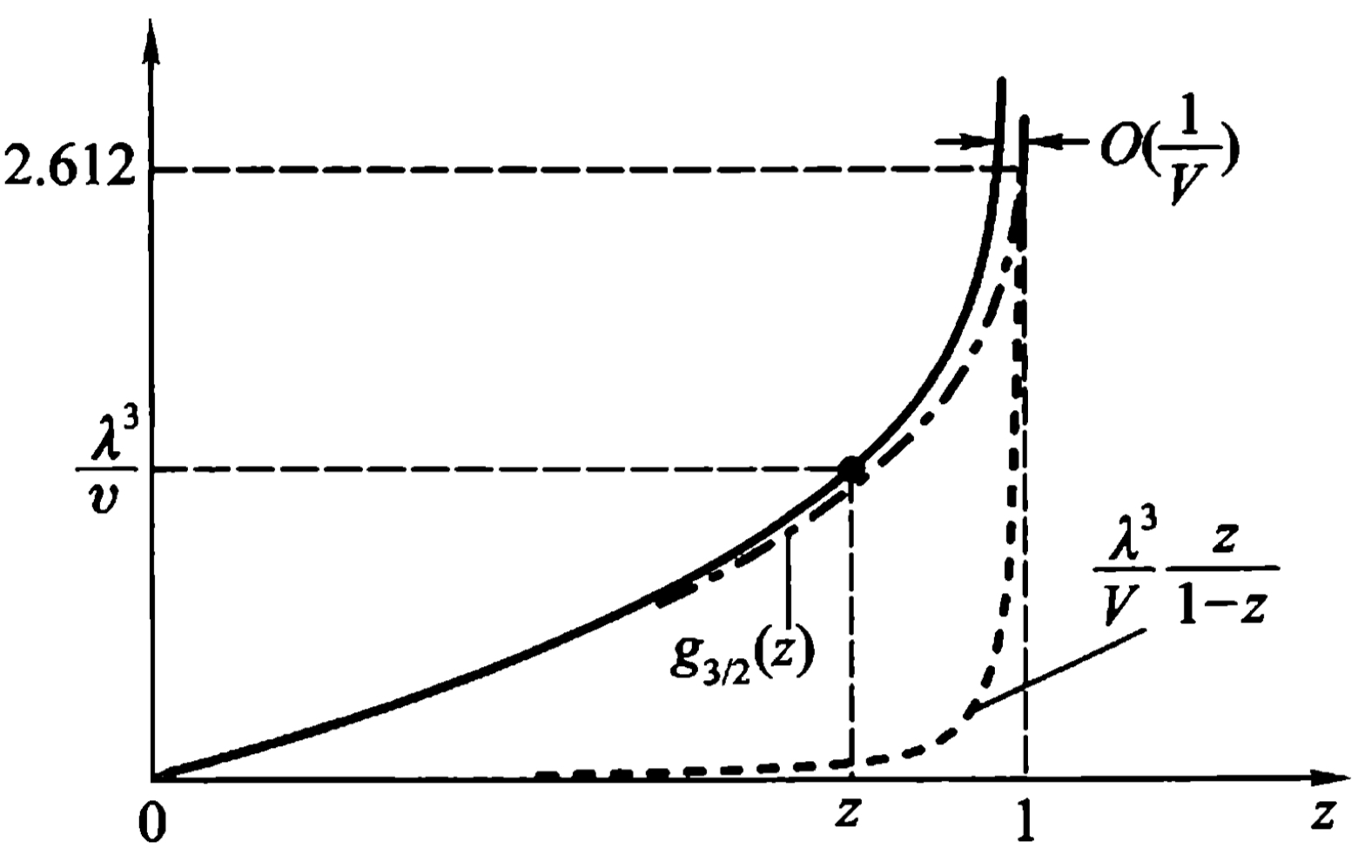
The equation of state of Bose gas is \begin{equation} \frac{P}{k_B T} = \frac{1}{\lambda^3} g_{5/2}(z)-\frac{1}{V}\ln (1-z) \end{equation} \begin{equation} \label{f3} \frac{1}{v} = \frac{1}{\lambda^3} g_{3/2}(z) + \frac{1}{V}\frac{z}{1-z} \end{equation} where \(v=\frac{V}{N}\), \(\lambda = \sqrt{\frac{2\pi \hbar^2}{mk_B T}}\). In formula \eqref{f3}, the function \(g_{3/2}(z)\) is \begin{equation} g_{3/2}(z)=\sum_{l=1}^{\infty} \frac{z^l}{l^{3/2}} \end{equation} For small \(z\) it can be written into: \begin{equation} g_{3/2}(z)=z+\frac{z^2}{2^{3/2} +\frac{z^3}{3^{3/2}}+\cdot \cdot \cdot} \end{equation} When \(z = 1\), its differential is divergent, while itself convergent to \begin{equation} g_{3/2}(1)=\sum_{l=1}^{\infty} \frac{1}{l^{3/2}} = \zeta (3/2) = 2.612\cdot \cdot \cdot \end{equation} Thus for all \(0 < z < 1\), we have \begin{equation} g_{3/2}(z)<2.612\cdot\cdot\cdot \end{equation}
Conditions of Bose-Einstein Condensation
We can rewrite the formula \eqref{f3}: \begin{equation} \label{f4} \lambda^3 \frac{\langle n_0 \rangle}{V} = \frac{\lambda^3}{v} - g_{3/2}(z) \end{equation} where \(\langle n_0 \rangle = \frac{z}{1-z}\) which represent the average occupation number of 0 momentum particles.
The formula \eqref{f4} means that if the temperature and specific volume meet: \begin{equation} \label{condition} \frac{\lambda^3}{v}>g_{3/2}(1) \end{equation} we have \begin{equation} \frac{\langle n_0 \rangle}{V}>0 \end{equation} which means BEC occurs. So the formula \eqref{condition} is the condition of BEC.
If the specific volume is fixed, we have the critical temperature \(T_c\): \begin{equation} k_B T_c=\frac{2\pi\hbar^2}{m[vg_{3/2}(1)]^{2/3}} \end{equation}
If the temperature is fixed, we have the critical specific volume \(v_c\): \begin{equation} v_c=\frac{\lambda^3}{g_{3/2}(1)} \end{equation} BEC only happens in systems that have high density and low temperature.
Fugacity
From figure we can know that \(z=1\) for all \(\frac{\lambda^3}{v}>g_{3/2}(1)\). When \(V \to \infty\), the fugacity meet: \begin{equation} z=1\text{, }(\frac{\lambda^3}{v}>g_{3/2}(1)) \end{equation} \begin{equation} \label{f5} g_{3/2}(z) = \frac{\lambda^3}{v} \text{, }(\frac{\lambda^3}{v}<g_{3/2}(1)) \end{equation}
Occupation number
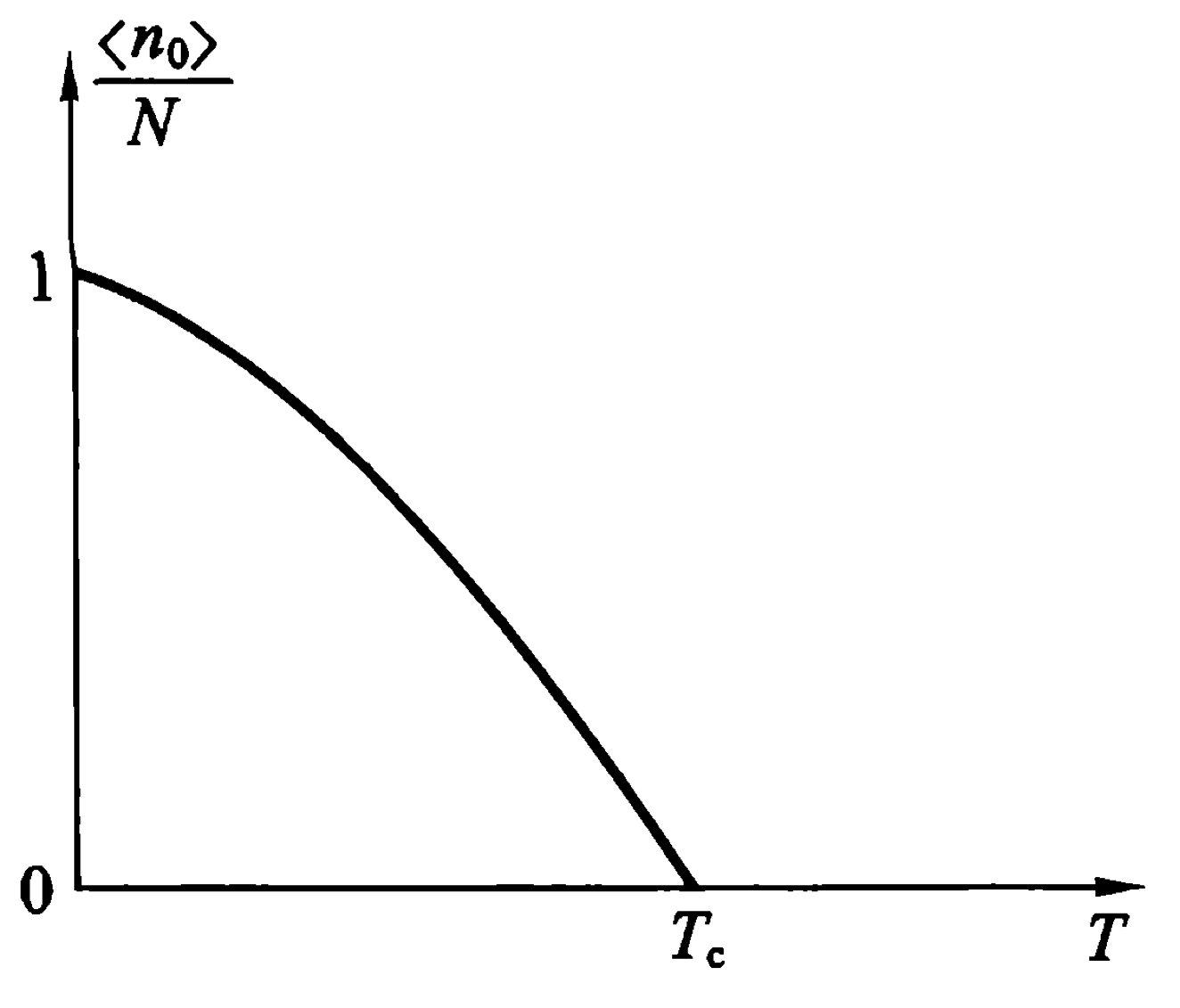
From equation \eqref{f5}, we can write the relation between \(\frac{\langle n_0 \rangle}{N}\) and Temperature or specific volume. \begin{equation} \frac{\langle n_0\rangle}{N} = 1-(\frac{T}{T_c})^{3/2} = 1-\frac{v}{v_c}\text{, }(\frac{\lambda^3}{v}>g_{3/2}(1)) \end{equation} \begin{equation} \frac{\langle n_0\rangle}{N} = 0\text{, }(\frac{\lambda^3}{v}<g_{3/2}(1)) \end{equation} The partition of particles with \(p=0\) is showed in the figure.
Equation of States
In the condensed area, the EOS is \begin{equation} \frac{P}{k_B T} = \frac{1}{\lambda^3}g_{5/2}(1)\text{, }(v<v_c) \end{equation} where \(g_{5/2}(1)=\zeta(5/2)=1.342\cdot\cdot\cdot\).
Curve of Phase Transition
In \(P-v\) diagram, the curve of phase transision is: \begin{equation} Pv^{5/3}=\frac{2\pi\hbar^2}{m}\frac{g_{5/2/}(1)}{[g_{3/2}(1)]^{5/3}} \end{equation} In \(P-T\) diagram, the curve is: \begin{equation} P=(\frac{m}{2\pi\hbar^2}^{3/2}g_{5/2}(1)(k_B T)^{5/2}) \end{equation}
Thermodynamic Quantities in Condensed Area
In the condensed area \(v<v_c(T<T_c)\), we can calculate the thermodynamic quantities of the system.
Internal Energy
\begin{equation} \frac{U}{N} = \frac{3}{2} Pv=\frac{3}{2}\frac{k_B Tv}{\lambda^3}g_{5/2}(1) \end{equation}
Entropy
\begin{equation} \frac{S}{Nk_B} = \frac{5}{2}\frac{v}{\lambda^3}g_{5/2}(1) \end{equation}
Specific Capacity
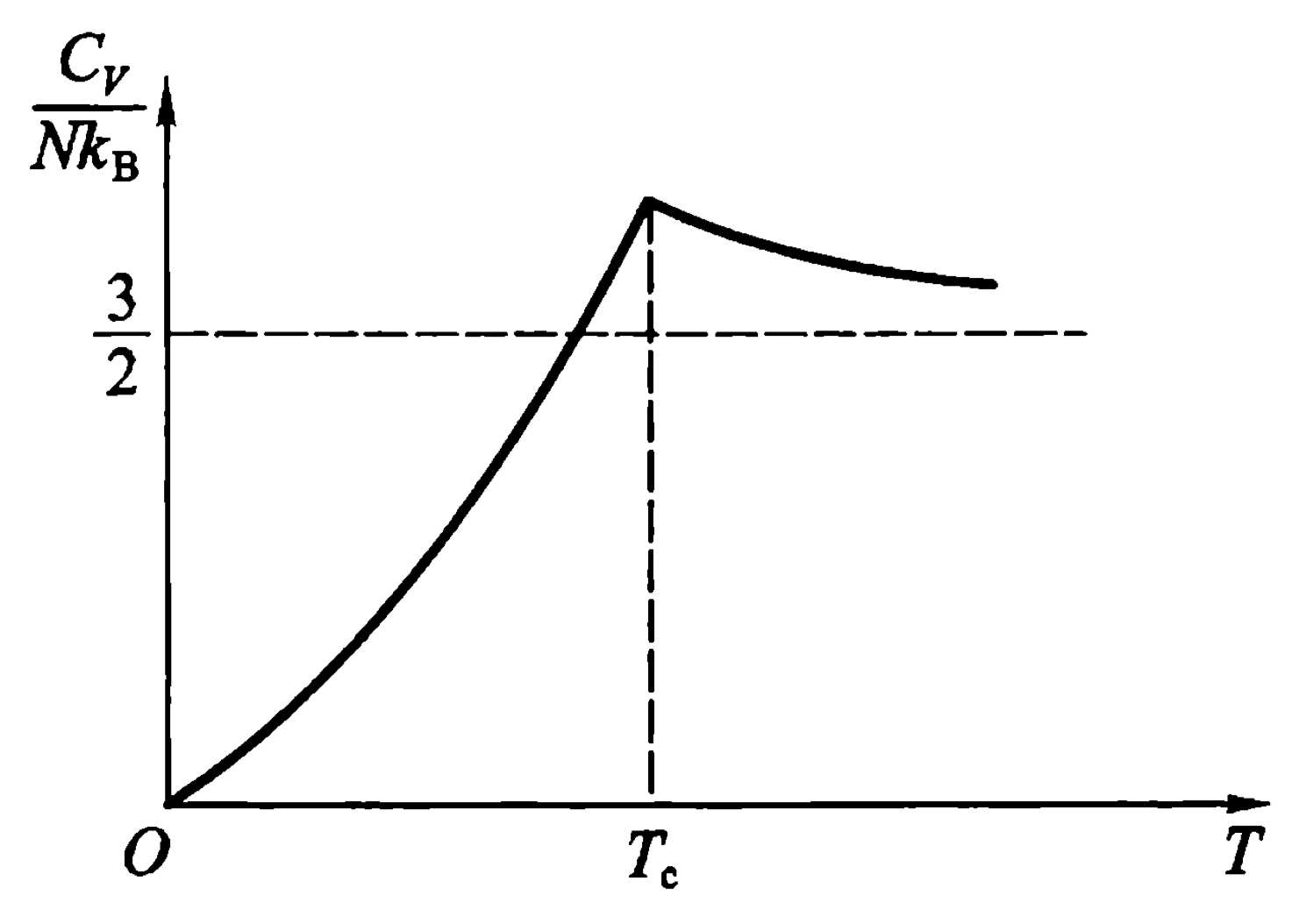
\begin{equation} \frac{C_v}{Nk_B} = \frac{15}{4}\frac{v}{\lambda^3}g_{5/2}(1) \end{equation} The figure of capacity is shown below.